What is coulomb’s force?
 |
| credit – shutterstock.com |
Notable points about coulomb’s force.
- Coulomb’s force depends on the magnitude of the charged bodies.
- It is an electrostatic force.
- S.I unit of coulomb’s force is Newton.
- Coulomb’s force is valid for stationary bodies.
- Coulomb’s force is vector quantity.
- Value of coulomb’s force can be negative, positive or zero.
- Coulomb’s force can be possible for at least two different charged bodies.
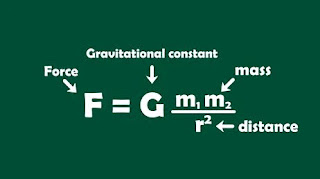
What is gravitational force?
 |
| credit – shutterstock |
Notable points on gravitational force
- Gravitational force depends on the magnitude of the uncharged bodies.
- It is not an electrostatic force.
- S.I unit of gravitational force is Newton.
- Gravitational force is valid for moving or stationary bodies.
- Gravitational force is vector quantity.
- Value of gravitational force can be negative, positive or zero.
- Gravitational force also can be possible for at least two different bodies.
Similarities between coulomb’s force and gravitational force
- Both Forces Coulomb’s and gravitational are directly proportional to the product of charges and masses of the body.
- Both Forces coulomb’s and gravitational are inversely proportional to the square of distance between them.
- Both forces are central forces.
- Both forces are conservative forces.
- Both forces can be calculated even in vaccum.
Coulomb’s force and gravitational force are both fundamental forces in nature that govern the interactions between objects. However, they differ in several key aspects. Below is a comparison that highlights their similarities and differences:
More similarities Between Coulomb’s Force and Gravitational Force
-
Inverse Square Law:
Both forces obey the inverse square law, meaning the magnitude of the force decreases with the square of the distance between the two interacting objects.- Coulomb’s Force:
- Gravitational Force:
Where: - are the charges,
- are the masses,
- is the distance between the objects,
- and are constants (Coulomb’s constant and the gravitational constant, respectively).
-
Action-at-a-Distance:
Both forces are long-range forces and act over a distance without requiring physical contact between the objects. They both affect objects even if they are not in direct contact. -
Central Forces:
Both forces are central forces, meaning they act along the line connecting the centers of the two interacting objects. -
Conservative Forces:
Both are conservative forces, meaning that the work done by these forces depends only on the initial and final positions, and not on the path taken. -
Force is Directly Proportional to the Magnitudes:
In both cases, the force is proportional to the product of the interacting quantities: - For Coulomb’s force, the force is proportional to the product of the charges ().
- For gravitational force, the force is proportional to the product of the masses ().
Differences between electrostatic force and gravitational force
- Electrostatic force can be the force of attraction or repulsion between two charges at rest, while gravitational force is the force of only attraction between two bodies.
- Coulomb’s force can attract or repel the objects but gravitational force can only attract the bodies not can repel.
- Electrostatic force (or coulomb force) depends on the nature of the medium while gravitational force doesn’t depends on the medium even it can be applicable in outer space.
- The value of electrostatic force changes as medium change but the value of gravitational force doesn’t changes as medium change.
- Electrostatic force has highly greater value than gravitational forces.
- Electrostatic force is very strong force while gravitational force is weak force as compared to coulomb force.
More dissimilarities Between Coulomb’s Force and Gravitational Force
| Property | Coulomb’s Force | Gravitational Force |
|---|---|---|
| Nature of Interaction | Acts between electric charges (positive or negative). | Acts between masses (always attractive). |
| Attraction or Repulsion | Attractive or Repulsive: Depending on the charges’ sign (like charges repel, opposite charges attract). | Always Attractive: Gravitational force always attracts masses towards each other. |
| Strength | Very Strong: Coulomb’s force is much stronger than gravity at the atomic and molecular levels. | Very Weak: Gravitational force is extremely weak compared to electromagnetic forces, especially at small scales. |
| Sign of the Force Constant | The force constant, , is positive, indicating both attraction and repulsion. | The force constant, , is positive, but the force is always attractive. |
| Range of Effect | Long-range, but can be neutralized if equal positive and negative charges are present. | Long-range but never neutralized (always acts, no cancellation). |
| Dependence on Medium | Coulomb’s force depends on the medium in which charges are placed. The force is reduced in a dielectric medium. | Gravitational force is independent of the medium and only depends on mass and distance. |
| Force Constant | Coulomb’s constant is much larger than the gravitational constant (i.e., , ). | Gravitational constant is very small, which makes gravitational forces relatively weak compared to electrostatic forces. |
| Origin | Coulomb’s force originates from electric charges and is described by electromagnetic interaction. | Gravitational force originates from mass and is described by gravitational interaction. |
| Relative Magnitude | The electromagnetic force is significantly stronger than the gravitational force, especially at atomic and molecular scales. | The gravitational force is incredibly weak compared to electromagnetic force, especially at small scales. |
| Effect at Small Scales | At atomic or molecular scales, Coulomb’s force dominates the behavior of particles (e.g., electrons and protons). | At atomic or molecular scales, gravitational force is negligible compared to Coulomb’s force and does not influence particle behavior. |
| Mathematical Expression | ( F = frac{k_e | q_1 q_2 |
Key Takeaways:
- Coulomb’s force is stronger and can be both attractive and repulsive, acting between electric charges.
- Gravitational force is always attractive and acts between masses, but it is much weaker than Coulomb’s force.
- Gravitational force is significant on large scales (like celestial bodies), while Coulomb’s force dominates at small, atomic, and molecular scales.


